Vieworks (CEO Husik Kim), a medical and industrial imaging solutions specialist, announced on the 12th that its digital medical image acquisition and processing software, VXvue, has received premarket clearance (510K) from the U.S. Food and Drug Administration (FDA). With this approval, Vieworks can now supply an integrated system combining an X-ray detector and dedicated software to the North American market.
Vieworks has received FDA approval for its software, which includes an AI-based image processing algorithm. Specifically, it demonstrated equivalence with commercially available medical devices, quality control procedures, and a post-management system, all in compliance with strengthened review standards.
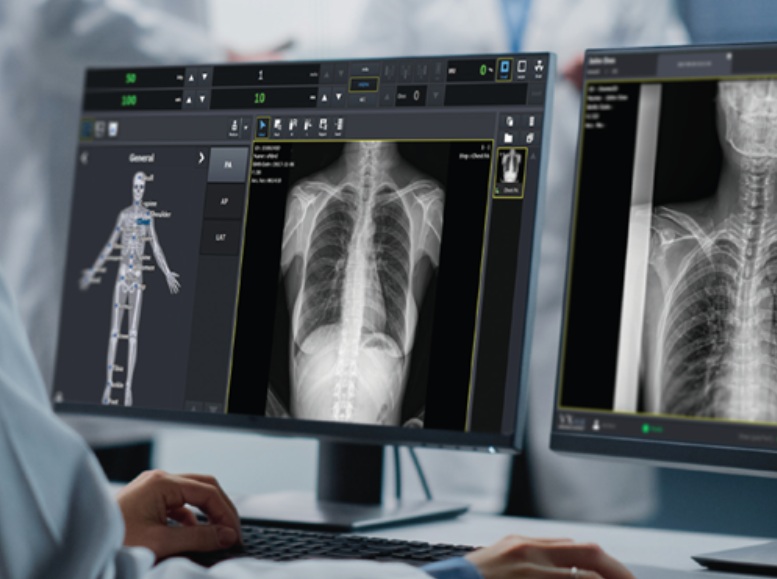
Our proprietary VXView is a user-centric software solution that transmits and processes clinical images acquired through an X-ray detector. Equipped with the "PureImpact™" algorithm, it enhances image clarity and enhances the detail of human soft tissue and skeletal structures. Furthermore, it offers presets tailored to specific clinical environments, including human, veterinary, and mobile examinations, enhancing user convenience.
Vieworks is strengthening its technological competitiveness by continuously securing global certifications, including this FDA approval and European CE certification. Last year, the long-type detector "VIVIX-S 1751S" and the mammography devices "VIVIX-M 1824S" and "VIVIX-M 2430S" received FDA approval. In May of this year, the slide scanner "VISQUE DPS LH510" received European in vitro diagnostic medical device certification "CE IVDR."
A Vieworks representative stated, “With this FDA approval, we have proven our global technological prowess in both hardware and software,” and added, “We will continue to develop high value-added solutions that meet the needs of the medical field, such as low-dose imaging, efficient data management, and strengthening the collaborative environment.”
- See more related articles
You must be logged in to post a comment.