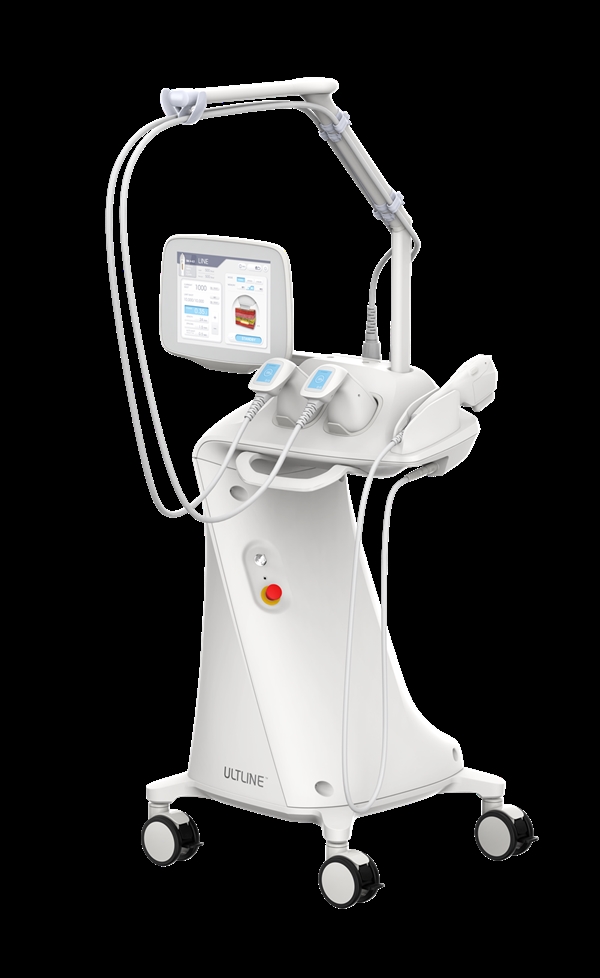
Eunsung Global (CEO Lee Ki-se) 's HIFU equipment 'ULTLINE' has obtained certification from the Brazilian National Agency for Food and Drug Surveillance (ANVISA). ANVISA certification is a prerequisite for entering the Brazilian market, and is granted only after passing strict quality standards and safety evaluations.
Ultrain is a device that helps reduce fat and improve skin elasticity based on high-intensity focused ultrasound (HIFU) technology. Ultrain can be used on various parts of the body through the 'Ultra Handpiece' for body slimming and the 'Line Handpiece' for face lifting. In particular, it is a great advantage that it can be customized to the patient's needs with various cartridges.
This acquisition of Ultrain ANVISA certification is an international recognition of Ultrain's technological prowess and stability, and it strengthens brand awareness and market share not only in Brazil but also in the entire Central and South American market.
It is expected to be an important stepping stone for expanding market share.
An official from Eunseong Global said, “Brazil is a key country in the Central and South American aesthetic medical device market, and obtaining this ANVISA certification will be an opportunity to further strengthen our competitiveness in the global market,” adding, “We will continue to introduce competitive new products through continuous technological innovation.”
- See more related articles
You must be logged in to post a comment.