Medical artificial intelligence (AI) company Lunit (CEO Seo Beom-seok) announced on the 15th that it has signed a supply contract with GC녹십자아이메드 for chest X-ray image analysis solution 'Lunit Insight CXR' and mammography image analysis solution 'Lunit Insight MMG'. Through this contract, Lunit plans to fully expand its business area into the health screening market.
According to this contract, the two Lunit Insights will be used at the Gangnam and Gangbuk hospitals of GC녹십자아이메드, a health screening institution under the GC녹십자 Medical Foundation. GC녹십자아이메드 is actively introducing the latest medical equipment and AI, and is evaluated as one of the leading screening institutions in Korea.
In particular, this contract is the first time that the Runit AI solution has been introduced as a non-covered item in a health screening environment. Until now, Runit Insight has only been used as a non-covered item in outpatient diagnosis areas such as general hospitals, but with its adoption as a non-covered item in the health screening market, it is expected to be an opportunity for AI-based medical technology to significantly expand in the screening area.
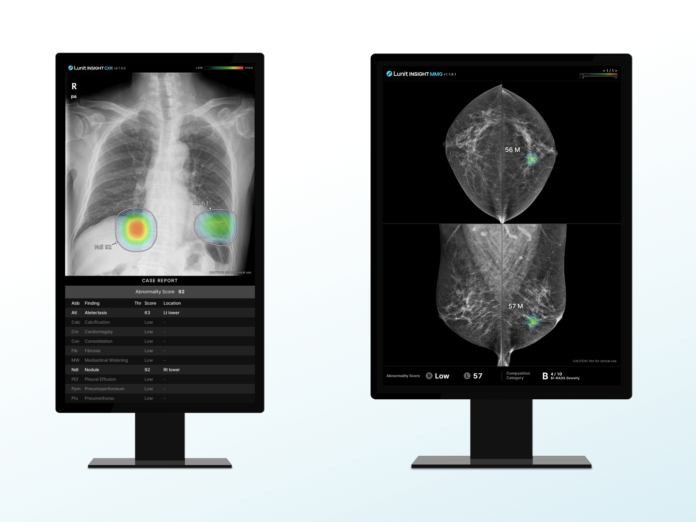
Additionally, when breast screening is conducted using Runit Insight MMG, examinees will receive an ‘AI Mammography Image Analysis Report’ along with a general health screening report.
The report includes information on the interpretation findings, and patient-tailored information such as breast cancer prevention methods and behavioral guidelines for additional examinations can also be checked via QR codes. This goes beyond the simple “normal” or “abnormal” results received from the examinee, and helps them clearly understand their health status and quickly decide on follow-up measures.
Seo Beom-seok, CEO of Lunit, said, “Lunit AI technology, which has proven its performance and utility through introduction in global medical institutions and various clinical studies, has now reached an important turning point for full-scale expansion into the screening environment.” He added, “This supply is particularly significant in that it is the first non-covered treatment application in the screening environment, and this will serve as an opportunity to further strengthen our position in the domestic market.”
- See more related articles
You must be logged in to post a comment.