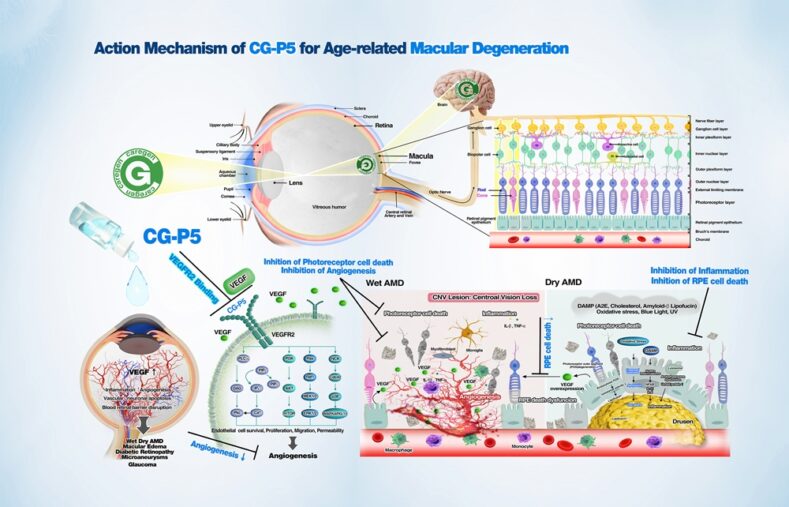
Caregen, a biotechnology company (CEO Yongji Jeong), will unveil its innovative wet macular degeneration treatment 'CG-P5' at the Asia Pacific Academy of Ophthalmology (APAO) 2025, which will be held in New Delhi, India from the 3rd to the 6th. This conference is the first international conference that Caregen is participating in in the field of ophthalmology, and is a prestigious event attended by many global ophthalmologists and pharmaceutical companies.
At APAO 2025, Caregen aims to introduce the mechanism of action and clinical progress of CG-P5 and strengthen its technological leadership and position in the global ophthalmology market. CG-P5 is an eye drop treatment based on a bioactive peptide. Unlike existing anti-VEGF injection treatments (e.g., Eylea, Lucentis, etc.), it can be administered as eye drops rather than directly injected into the eye, which is expected to dramatically improve patient convenience and compliance.
Currently, CG-P5 is in the final stages of the US FDA phase 1 clinical trial, and a comparative clinical trial using Eylea as a control group is in progress. The clinical trial is scheduled to end around June 2025, and an application for Breakthrough Therapy Designation (BTD) is being prepared thereafter. In addition, plans are being made to enter phase 2 clinical trials by expanding indications such as dry macular degeneration.
Based on this progress, active technology transfer discussions are ongoing with a number of global ophthalmology pharmaceutical companies, and in particular, discussions with pharmaceutical companies in major countries including China and India are progressing considerably. Caregen expects that full-scale global partnerships will become visible starting with APAO 2025.
Caregen CEO Yong-ji Jeong emphasized, “CG-P5 is an innovative peptide-based eye drop treatment that overcomes the limitations of existing treatments. We will strengthen strategic cooperation with global pharmaceutical companies and accelerate our market entry at APAO 2025.” He also added, “The ongoing technology transfer proposals are examples that prove the high marketability and technological excellence of CG-P5.”
Meanwhile, Caregen is also focusing its capabilities on developing 'CG-T1', a dry eye treatment, in addition to the wet macular degeneration treatment. Unlike existing cyclosporine preparations, CG-T1 is a peptide-based drug that targets the PAC1 receptor, and is expected to have a more fundamental and rapid treatment effect as it can effectively suppress inflammation while minimizing eye irritation when instilled. Caregen is preparing to apply for an investigational new drug (IND) to the US FDA for phase 1 clinical trials in the second half of 2025.
Caregen plans to disclose the action mechanisms and research data of CG-P5 and CG-T1 at APAO 2025 and strengthen its collaborative network with ophthalmology experts and researchers from around the world. In addition, starting with APAO, the company plans to continuously participate in major international ophthalmology conferences and exhibitions, such as the European Society of Retina (EURETINA), to gradually expand its presence and influence in the global ophthalmology market.
- See more related articles
You must be logged in to post a comment.