COMEUP 2020 raised its curtain on November 19th. Chun Jong Yoon, the CEO of Seegene who developed the COVID-19 diagnostic kit, and spread the excellence of K-quarantine, shared his experience of overcoming the COVID-19 crisis as a speaker of the main conference topic.
South Korea is being a great example to other countries as a model country for COVID-19 quarantine, and in the middle of it stands the diagnostic kit of Seegene. Chun, in the session, said, “The diagnostic kit technique, central testing system, and mass testing played big roles in South Korea’s efficiency in the response to the COVID-19 outbreak.”
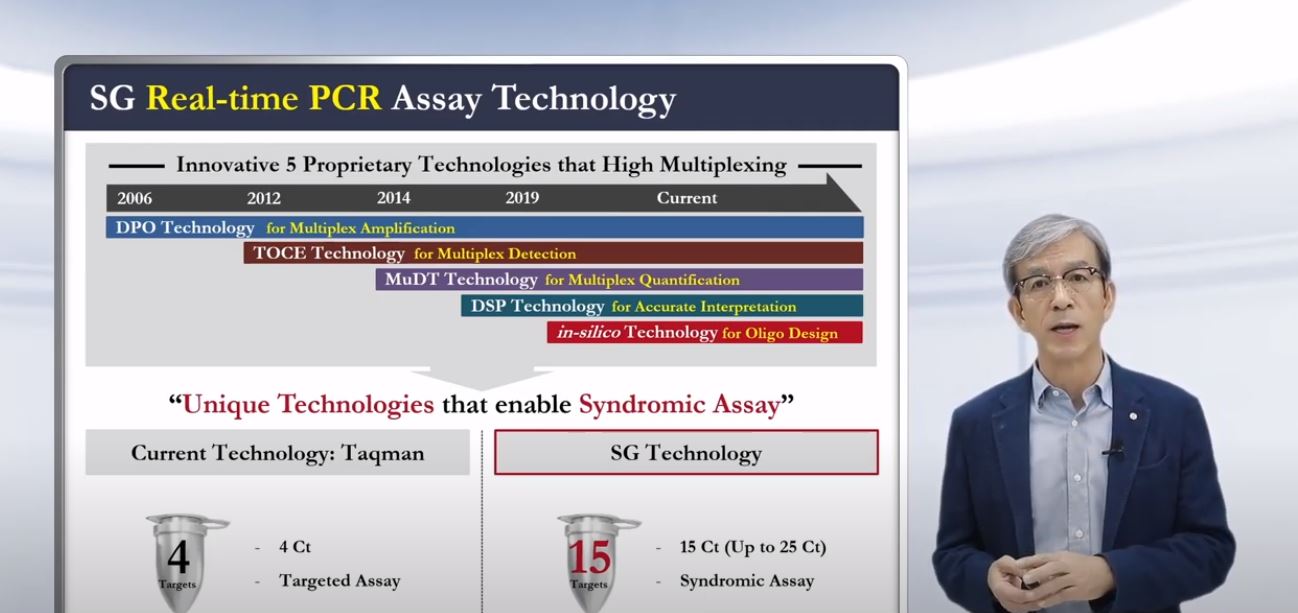
First of all, a characteristic of Seegene’s diagnostic kit is the development method of applying an automated system and using digital techniques and AI. Based on such techniques, the company developed its COVID-19 diagnostic kit in 2 weeks and received an emergency use approval in a month. A diagnostic kit of another company could only perform 1-2 genetic tests using a single tube, but Seegene’s kit allows up to 3 tests at once. In addition, Chun explained that bringing the central testing system into each hospital allowed mass testing and fast obtainment of results.
Chun added that “our country was successful in quarantine because of the recognition that the diagnosis speed has to be faster than the speed of COVID-19 spreading,” and said that the key to controlling the spreading of the virus is in the diagnosis speed. When dividing the confirmed cases in South Korea by the number of cumulative tests, the country’s positive rate is around 1%. Chun explained that this number is very low compared to other countries.
With these technical skills, Seegene released a new diagnostic kit last May. The new kit, with an increase from 3 to 4 genetic test targets compared to before, was released as the company’s existing kit started to incur suspicion due to the emergence of a COVID-19 variant. It requires no extraction process so it also led to reduced time and cost.
Chun stated that such a technique is “only possessed by Seegene” and showed confidence that their kit is “the only product that can test not only COVID-19 genetic information but also flu (influenza) and RSV (respiratory syncytial virus – general cold virus) at the same time.”
Lastly, Chun explained about changes that will be brought upon the wide-spreading of molecular diagnosis in the future. He said that “there will be more chances to get molecule-based diagnoses” and that “Seegene will make an automated platform for molecular diagnosis”. He also revealed his wish to draw forth molecular diagnosis in various fields such as marine and environment by sharing the platform with other industries.


You must be logged in to post a comment.